Thermodynamic Database
Components (38)
Total of 38 components are included in the database as listed here:
Major alloying elements: Al, Cu, Fe, Mg, Mn, Si and Zn
Minor alloying elements: Ag, B, Ba, Be, Bi, C, Ca, Ce, Co, Cr, Er, Gd, Ge, Hf, K, La, Li, Na, Nb, Ni, O, Pb, Sb, Sc, Sn, Sr, Ti, V, W, Y, and Zr
Suggested Composition Range
The suggested composition range for each element is listed in Table 1. It should be noted that this given composition range is rather conservative. It is derived from the chemistries of the multicomponent commercial alloys that have been used to validate the current database. In the subsystems, many of these elements can be applied to a much wider composition range. In fact, some binary and ternary systems have been fully assessed and they are covered in the full range from 0-100% as given in Section Assessed Subsystems.
| Elements | Composition Range (wt.%) |
|---|---|
|
Al |
80 ~ 100 |
|
Cu |
0 ~ 5.5 |
|
Fe |
0 ~ 2.0 |
|
Mg |
0 ~ 7.6 |
|
Mn |
0 ~ 1.2 |
|
Si |
0 ~ 17.5 |
|
Zn |
0 ~ 8.1 |
|
Others |
0 ~ 1 |
What is new in PanAl2024
-
Addition of the component O and the O-X binary systems
-
Update the stability of the Al15_FeMn3Si2 phase
-
Improved the La-B, and Al-Sc-Si systems
Phases
Total of 1150 phases are included in the current database. The names and thermodynamic models of some phases are given in Table 2. Information on all the other phases is listed in PanAl2024: List of Phases. Users can also view it through TDB viewer of Pandat™ .
Assessed Subsystems
A total of 537 subsystems, including 455 binary and 82 ternary subsystems have been assessed. The modeling status is indicated by numbers. The systems with number 10 are fully assessed in the whole composition range. The higher value shows higher reliability of the system.
Binary Systems (455)
| Ag-Al(10) | Ag-B(10) | Ag-Bi(10) | Ag-C(10) | Ag-Ca(10) | Ag-Ce(10) | Ag-Co(10) |
| Ag-Cr(10) | Ag-Cu(10) | Ag-Er(10) | Ag-Fe(10) | Ag-Gd(10) | Ag-Ge(10) | Ag-La(10) |
| Ag-Mg(10) | Ag-Na(10) | Ag-Ni(10) | Ag-O(5) | Ag-Pb(10) | Ag-Sb(10) | Ag-Sc(10) |
| Ag-Si(10) | Ag-Sn(10) | Ag-Sr(10) | Ag-Ti(10) | Ag-V(10) | Ag-W(10) | Ag-Y(10) |
| Ag-Zn(10) | Ag-Zr(10) | Al-B(10) | Al-Ba(10) | Al-Be(10) | Al-Bi(10) | Al-C(10) |
| Al-Ca(10) | Al-Ce(10) | Al-Co(10) | Al-Cr(10) | Al-Cu(10) | Al-Er(10) | Al-Fe(10) |
| Al-Gd(10) | Al-Ge(10) | Al-Hf(10) | Al-K(10) | Al-La(10) | Al-Li(10) | Al-Mg(10) |
| Al-Mn(10) | Al-Na(10) | Al-Nb(10) | Al-Ni(10) | Al-O(10) | Al-Pb(10) | Al-Sb(10) |
| Al-Sc(10) | Al-Si(10) | Al-Sn(10) | Al-Sr(10) | Al-Ti(10) | Al-V(10) | Al-W(10) |
| Al-Y(10) | Al-Zn(10) | Al-Zr(10) | B-C(10) | B-Ca(10) | B-Co(10) | B-Cr(10) |
| B-Cu(10) | B-Er(10) | B-Fe(10) | B-Hf(10) | B-La(10) | B-Mg(10) | B-Mn(10) |
| B-Nb(10) | B-Ni(10) | B-O(10) | B-Sc(10) | B-Si(10) | B-Sn(10) | B-Sr(10) |
| B-Ti(10) | B-V(10) | B-W(10) | B-Zr(10) | Ba-Be(10) | Ba-Bi(10) | Ba-Ca(10) |
| Ba-Cr(10) | Ba-Cu(10) | Ba-Fe(10) | Ba-Gd(10) | Ba-Ge(10) | Ba-La(10) | Ba-Li(10) |
| Ba-Mg(10) | Ba-Mn(10) | Ba-Ni(10) | Ba-O(10) | Ba-Pb(10) | Ba-Sc(10) | Ba-Si(10) |
| Ba-Sr(10) | Ba-Ti(10) | Ba-V(10) | Ba-Y(10) | Ba-Zn(10) | Be-O(10) | Be-Si(10) |
| Bi-Cr(10) | Bi-Cu(10) | Bi-Er(10) | Bi-Fe(10) | Bi-Gd(10) | Bi-Ge(10) | Bi-La(10) |
| Bi-Mg(10) | Bi-Mn(10) | Bi-Ni(10) | Bi-O(10) | Bi-Pb(10) | Bi-Sb(10) | Bi-Si(10) |
| Bi-Sn(10) | Bi-Ti(10) | Bi-Zn(10) | C-Ce(10) | C-Co(10) | C-Cr(10) | C-Fe(10) |
| C-Ge(10) | C-Li(10) | C-Mg(10) | C-Mn(10) | C-Nb(10) | C-Ni(10) | C-O(10) |
| C-Si(10) | C-V(10) | C-W(10) | C-Zr(10) | Ca-Ce(10) | Ca-Cu(10) | Ca-Er(10) |
| Ca-Fe(10) | Ca-Gd(10) | Ca-La(10) | Ca-Li(10) | Ca-Mg(10) | Ca-Mn(10) | Ca-Na(10) |
| Ca-Ni(10) | Ca-O(10) | Ca-Pb(10) | Ca-Sc(10) | Ca-Si(10) | Ca-Sn(10) | Ca-Sr(10) |
| Ca-Zn(10) | Ce-Co(10) | Ce-Cu(10) | Ce-Er(10) | Ce-Fe(10) | Ce-La(10) | Ce-Li(10) |
| Ce-Mg(10) | Ce-Mn(10) | Ce-Ni(10) | Ce-O(10) | Ce-Sb(10) | Ce-Sc(10) | Ce-Si(10) |
| Ce-Ti(10) | Ce-V(10) | Ce-Y(10) | Ce-Zn(10) | Co-Cr(10) | Co-Cu(10) | Co-Er(10) |
| Co-Fe(10) | Co-Ge(10) | Co-La(10) | Co-Mn(10) | Co-Nb(10) | Co-Ni(10) | Co-O(10) |
| Co-Sb(10) | Co-Sc(10) | Co-Si(10) | Co-Sn(10) | Co-W(10) | Co-Y(10) | Co-Zn(10) |
| Cr-Cu(10) | Cr-Fe(10) | Cr-Ge(10) | Cr-Hf(10) | Cr-La(10) | Cr-Mg(10) | Cr-Mn(10) |
| Cr-Na(10) | Cr-Nb(10) | Cr-Ni(10) | Cr-O(10) | Cr-Sc(10) | Cr-Si(10) | Cr-Sn(10) |
| Cr-Ti(10) | Cr-V(10) | Cr-W(10) | Cr-Y(10) | Cr-Zn(10) | Cr-Zr(10) | Cu-Er(10) |
| Cu-Fe(10) | Cu-Gd(10) | Cu-Ge(10) | Cu-Hf(10) | Cu-La(10) | Cu-Li(10) | Cu-Mg(10) |
| Cu-Mn(10) | Cu-Na(10) | Cu-Nb(10) | Cu-Ni(10) | Cu-O(10) | Cu-Pb(10) | Cu-Sb(10) |
| Cu-Sc(10) | Cu-Si(10) | Cu-Sn(10) | Cu-Sr(10) | Cu-Ti(10) | Cu-V(10) | Cu-W(10) |
| Cu-Y(10) | Cu-Zn(10) | Cu-Zr(10) | Er-Fe(10) | Er-Gd(10) | Er-Ge(10) | Er-Hf(10) |
| Er-La(10) | Er-Li(10) | Er-Mg(10) | Er-Mn(10) | Er-Nb(10) | Er-O(5) | Er-Sc(10) |
| Er-Si(10) | Er-Ti(10) | Er-V(10) | Er-W(10) | Er-Y(10) | Er-Zn(10) | Er-Zr(10) |
| Fe-Gd(10) | Fe-Hf(10) | Fe-La(10) | Fe-Mg(10) | Fe-Mn(10) | Fe-Nb(10) | Fe-Ni(10) |
| Fe-O(10) | Fe-Pb(10) | Fe-Sb(10) | Fe-Sc(10) | Fe-Si(10) | Fe-Sn(10) | Fe-Sr(10) |
| Fe-Ti(10) | Fe-V(10) | Fe-W(10) | Fe-Y(10) | Fe-Zn(10) | Fe-Zr(10) | Gd-Mg(10) |
| Gd-Mn(10) | Gd-Ni(10) | Gd-O(5) | Gd-Sb(10) | Gd-Sc(10) | Gd-Si(10) | Gd-Ti(10) |
| Gd-Zn(10) | Gd-Zr(10) | Ge-Hf(10) | Ge-K(10) | Ge-Mg(10) | Ge-Mn(10) | Ge-Na(10) |
| Ge-Nb(10) | Ge-Ni(10) | Ge-O(10) | Ge-Pb(10) | Ge-Sb(10) | Ge-Sc(10) | Ge-Si(10) |
| Ge-Sn(10) | Ge-Sr(10) | Ge-Ti(10) | Ge-V(10) | Ge-W(8) | Ge-Zn(10) | Hf-Mn(10) |
| Hf-Nb(10) | Hf-Ni(10) | Hf-O(10) | Hf-Si(10) | Hf-Sn(10) | Hf-Ti(10) | Hf-W(10) |
| Hf-Zr(10) | K-Na(10) | K-O(5) | La-Mg(10) | La-Mn(10) | La-Nb(10) | La-Ni(10) |
| La-O(5) | La-Pb(10) | La-Sb(10) | La-Sc(10) | La-Si(10) | La-Sn(10) | La-Ti(10) |
| La-V(10) | La-W(10) | La-Zn(10) | La-Zr(10) | Li-Mg(10) | Li-Mn(10) | Li-Na(10) |
| Li-O(10) | Li-Sc(10) | Li-Si(10) | Li-Sn(10) | Li-Sr(10) | Li-Zn(10) | Li-Zr(10) |
| Mg-Mn(10) | Mg-Na(10) | Mg-Ni(10) | Mg-O(10) | Mg-Sb(10) | Mg-Sc(10) | Mg-Si(10) |
| Mg-Sn(10) | Mg-Sr(10) | Mg-Ti(10) | Mg-Y(10) | Mg-Zn(10) | Mg-Zr(10) | Mn-Nb(10) |
| Mn-Ni(10) | Mn-O(10) | Mn-Pb(10) | Mn-Sc(10) | Mn-Si(10) | Mn-Sn(10) | Mn-Sr(10) |
| Mn-Ti(10) | Mn-V(10) | Mn-W(10) | Mn-Y(10) | Mn-Zn(10) | Mn-Zr(10) | Na-O(7) |
| Na-Si(10) | Na-Sr(10) | Na-Zn(10) | Nb-Ni(10) | Nb-O(10) | Nb-Si(10) | Nb-Sn(10) |
| Nb-Sr(10) | Nb-Ti(10) | Nb-V(10) | Nb-W(10) | Nb-Y(10) | Nb-Zr(10) | Ni-O(10) |
| Ni-Pb(10) | Ni-Sb(10) | Ni-Si(10) | Ni-Sn(10) | Ni-Sr(10) | Ni-Ti(10) | Ni-V(10) |
| Ni-W(10) | Ni-Y(10) | Ni-Zn(10) | Ni-Zr(10) | O-Pb(10) | O-Sb(7) | O-Sc(5) |
| O-Si(10) | O-Sn(10) | O-Sr(5) | O-Ti(10) | O-V(10) | O-W(10) | O-Y(10) |
| O-Zn(5) | O-Zr(10) | Pb-Sb(10) | Pb-Si(10) | Pb-Sn(10) | Pb-Zn(10) | Pb-Zr(10) |
| Sb-Si(10) | Sb-Sn(10) | Sb-Zn(10) | Sc-Si(10) | Sc-Sn(10) | Sc-Sr(10) | Sc-Ti(10) |
| Sc-W(10) | Sc-Y(10) | Sc-Zn(10) | Sc-Zr(10) | Si-Sn(10) | Si-Sr(10) | Si-Ti(10) |
| Si-V(10) | Si-W(10) | Si-Y(10) | Si-Zn(10) | Si-Zr(10) | Sn-Sr(10) | Sn-Ti(10) |
| Sn-V(10) | Sn-W(10) | Sn-Y(10) | Sn-Zn(10) | Sn-Zr(10) | Sr-Ti(10) | Sr-V(10) |
| Sr-Y(10) | Sr-Zn(10) | Ti-V(10) | Ti-W(10) | Ti-Zr(10) | V-W(10) | V-Y(10) |
| V-Zn(10) | V-Zr(10) | W-Y(10) | W-Zr(10) | Y-Zn(10) | Y-Zr(10) | Zn-Zr(10) |
Ternary Systems (82)
| Ag-Al-Cu(10) | Ag-Al-Ge(5) | Ag-Al-Mg(10) | Ag-Al-Si(10) | Ag-Al-Zn(5) | Ag-Cu-Mg(10) |
| Al-B-Fe(8) | Al-Ba-Ce(8) | Al-Ba-Fe(8) | Al-Ba-La(5) | Al-Ba-Mn(5) | Al-Ba-Si(10) |
| Al-Ba-Y(5) | Al-Be-Si(5) | Al-C-Zr(10) | Al-Ca-Mg(10) | Al-Ca-Mn(5) | Al-Ce-Co(5) |
| Al-Ce-Cu(5) | Al-Ce-Fe(10) | Al-Ce-Mg(10) | Al-Ce-Mn(8) | Al-Ce-Si(8) | Al-Co-Cu(10) |
| Al-Co-Fe(10) | Al-Co-Mn(5) | Al-Co-Si(10) | Al-Cr-Fe(10) | Al-Cr-Mn(5) | Al-Cr-Ni(10) |
| Al-Cr-Si(10) | Al-Cu-Er(5) | Al-Cu-Fe(10) | Al-Cu-Li(10) | Al-Cu-Mg(10) | Al-Cu-Mn(5) |
| Al-Cu-Sb(5) | Al-Cu-Sc(5) | Al-Cu-Si(10) | Al-Cu-Zn(10) | Al-Er-Fe(5) | Al-Er-Mg(10) |
| Al-Er-Mn(5) | Al-Fe-La(8) | Al-Fe-Mg(10) | Al-Fe-Mn(10) | Al-Fe-Ni(10) | Al-Fe-Si(10) |
| Al-Fe-Y(8) | Al-Fe-Zn(10) | Al-Gd-Mn(8) | Al-Gd-Ni(5) | Al-Ge-Si(5) | Al-La-Mg(5) |
| Al-La-Mn(5) | Al-La-Si(10) | Al-La-Y(5) | Al-Li-Mg(10) | Al-Li-Si(10) | Al-Li-Zn(10) |
| Al-Mg-Mn(5) | Al-Mg-Na(5) | Al-Mg-Sb(5) | Al-Mg-Sc(5) | Al-Mg-Si(10) | Al-Mg-V(10) |
| Al-Mg-Y(5) | Al-Mg-Zn(10) | Al-Mn-Si(10) | Al-Mn-Y(5) | Al-Si-Sn(5) | Al-Si-Sr(10) |
| Al-Si-Ti(10) | Al-Si-Y(5) | Al-Si-Zn(10) | Al-Si-Zr(10) | Bi-Sn-Zn(10) | Cu-Mg-Si(10) |
| Cu-Mg-Zn(10) | Fe-Mn-Si(5) | Li-Mg-Si(10) | Mg-Si-Zn(5) |
Database Validation
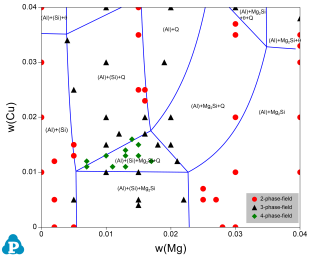
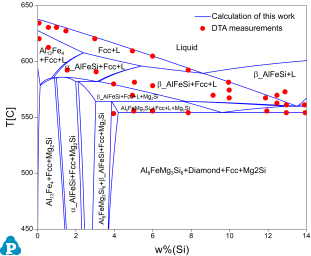
The PanAl database is validated by a large amount of phase equilibrium data. Two examples are shown here. Figure 1 shows the calculated isotherm of Al-Cu-Mg-Si at 500ºC with Si content of 1.8wt%. The experimental data of D.P. Smith [1962Smi] are plotted on it for comparison. Figure 2 shows the calculated isopleth of Al-Fe-Mg-Si at 4wt.%Mg and 0.5wt.%Fe with experimental data from Phillips [1961Phi].
Figure 1: Comparison of a calculated isothermal section of Al-Cu-Mg-Si at 1.8wt%Si and at T=500 °C with the experimental data [1962Smi]
Figure 2: Comparison of a calculated isopleth of Al-Fe-Mg-Si at 4wt.%Mg and 0.5wt.%Fe with the experimental data [1961Phi]
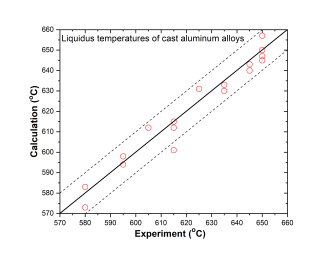
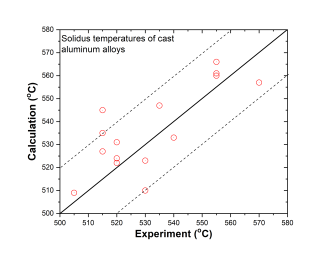
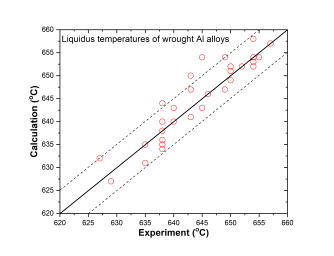
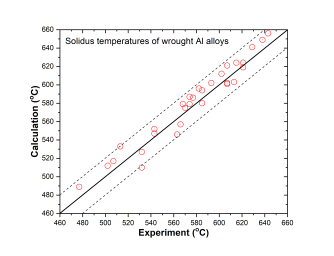
In addition to the validation of phase equilibria, the current database has also been subjected to extensive validation by the solidification data of commercial aluminum alloys. The predicted liquidus and solidus temperatures of cast and wrought are shown in Figure 3 ~ Figure 6 with experimental data, respectively.
Cast aluminum alloys: 201, 206, 208, 242, 295, 296, 308, 319, 356, 357, 359, 360, 380, 383, 384, 390, 771, 850
Wrought aluminum alloys: 2014, 2017, 2024, 2036, 2124, 2218, 2219, 2319, 3003, 3004, 3105, 4032, 5052, 5056, 5083, 5086, 5154, 5182, 5356, 5454, 5456, 5457, 6005, 6009, 6010, 6061, 6063, 6066, 6070, 6101, 6151, 6201, 6205, 6351, 6463, 7005, 7039, 7049, 7075, 7178, 7475
Figure 3: Comparison between calculated and experimentally measured liquidus temperatures of cast aluminum alloys
Figure 4: Comparison between calculated and experimentally measured solidus temperatures of cast aluminum alloys
Figure 5: Comparison between calculated and experimentally measured liquidus temperatures of wrought aluminum alloys
Figure 6: Comparison between calculated and experimentally measured solidus temperatures of wrought aluminum alloys
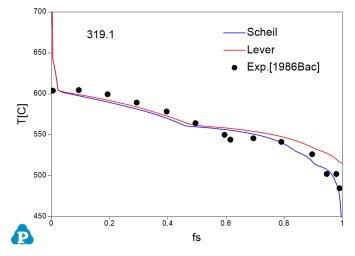
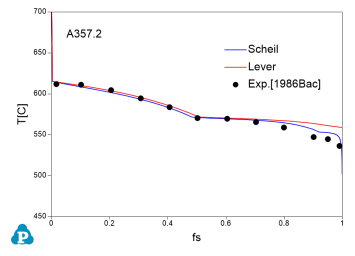
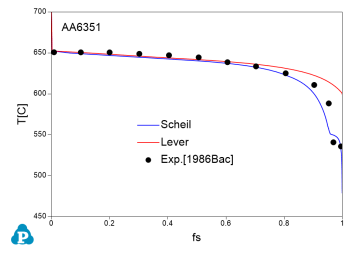
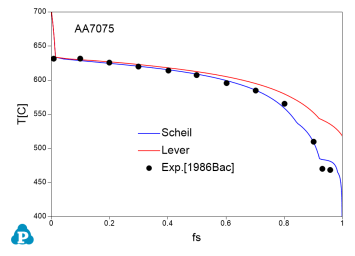
The solidification paths of several commercial aluminum alloys were experimentally investigated by Backerud et al [1986Bac]. The simulated solidification paths using both Scheil model and lever rule for four of these alloys (319.1, A357.2, A6351, A7075) are shown in Figure 7 ~ Figure 10 with the experimental data of Backerud et al [1986Bac].
Figure 7: Comparison between calculated and experimentally measured solidification paths of 319.1 aluminum alloy
Figure 8: Comparison between calculated and experimentally measured solidification paths of A357.2 aluminum alloy
Figure 9: Comparison between calculated and experimentally measured solidification paths of AA6351 aluminum alloy
Figure 10: Comparison between calculated and experimentally measured solidification paths of AA7075 aluminum alloy
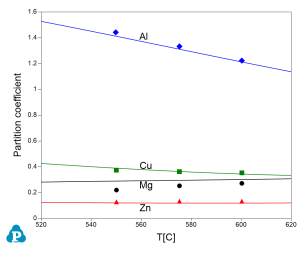
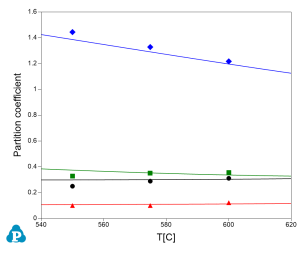
This database can also supply important parameters for processing simulation. One of these parameters is partition coefficient. The calculated partition coefficients have also been extensively validated. Examples for two Al-Cu-Mg-Zn quaternary alloys are given below. Figure 11 and Figure 12 show comparisons between calculated and measured partition coefficient for Al-4Cu-0.9Mg-2.6Zn (wt%) and Al-2.5Cu-1.3Mg-2.63Zn (wt%), respectively. The good agreement between the experimental and calculated results, as shown in these figures, indicates the reliability of the current PanAl thermodynamic database in providing thermodynamic input for processing simulation.
Figure 11: Calculated and experimentally determined [1998Lia] partition coefficients of Al-4Cu-0.9Mg-2.6Zn (wt%) alloy
Figure 12: Calculated and experimentally determined [1998Lia] partition coefficients of Al-2.5Cu-1.3Mg-2.63Zn (wt%) alloy
[1961Phi] H.W.L. Phillips, Equilibrium diagrams of aluminum alloy systems, The aluminum development association, Information Bulletin 25, London, (1961): 105-108.
[1962Smi] D.P. Smith, Metallurgia, 1(1962): 223-229.
[1986Bac] L.Backerud, et al., Solidification Characteristics of Aluminum Alloys, (1986), Oslo: Tangen Trykk A/S.
[1998Lia] P. Liang, et al., Experimental investigation and thermodynamic calculation of the Al-Mg-Zn system, Thermochim. Acta, 314 (1998): 87-110.